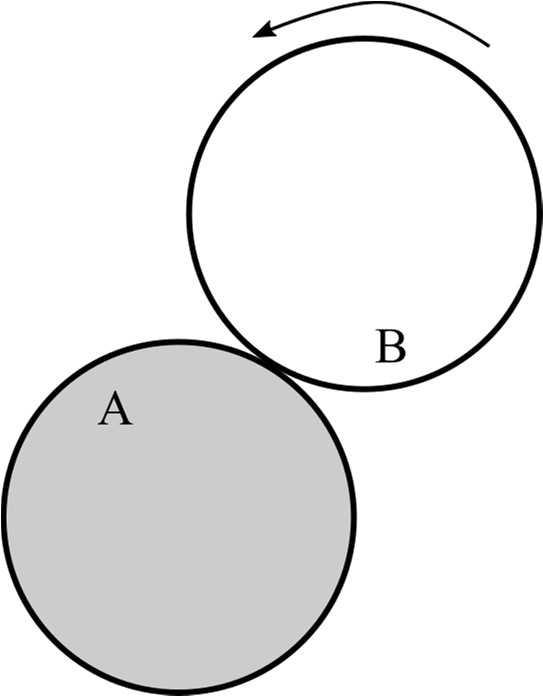
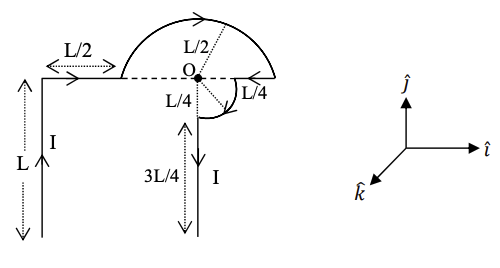
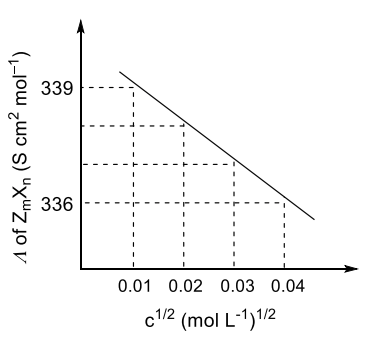
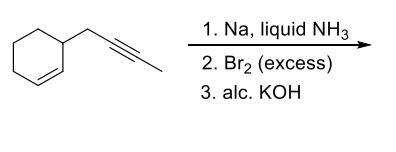
Q.1
Let $\alpha$ and $\beta$ be real numbers such that $-\frac{\pi}{4} < \beta < 0 < \alpha <
\frac{\pi}{4}$. If $\sin(\alpha + \beta)=\frac{1}{3}$ and $\cos(\alpha -
\beta)=\frac{2}{3}$, then the greatest integer less than or equal to $$ \left(
\frac{\sin \alpha}{\cos \beta} + \frac{\cos \beta}{\sin \alpha} + \frac{\cos
\alpha}{\sin \beta} + \frac{\sin \beta}{\cos \alpha} \right)^2 $$ is _____________.
1
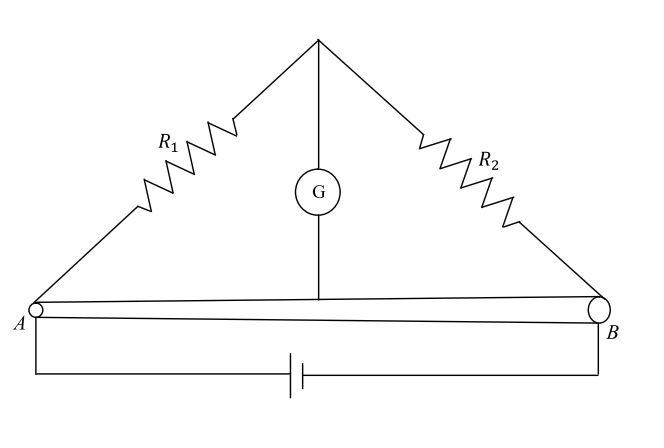
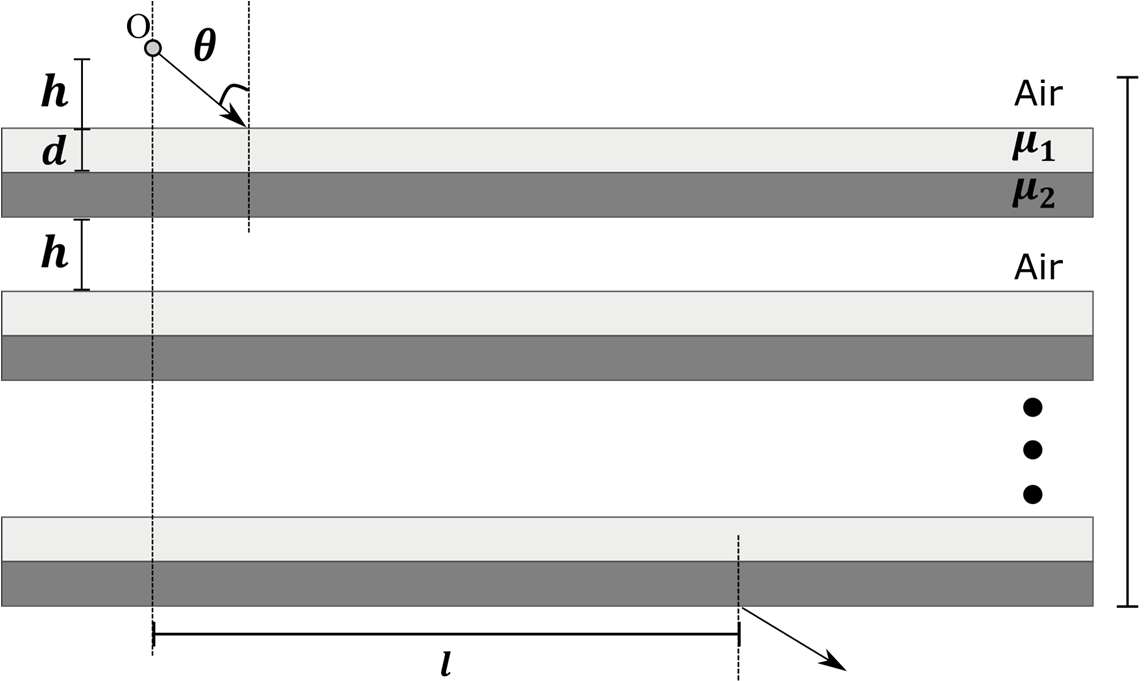
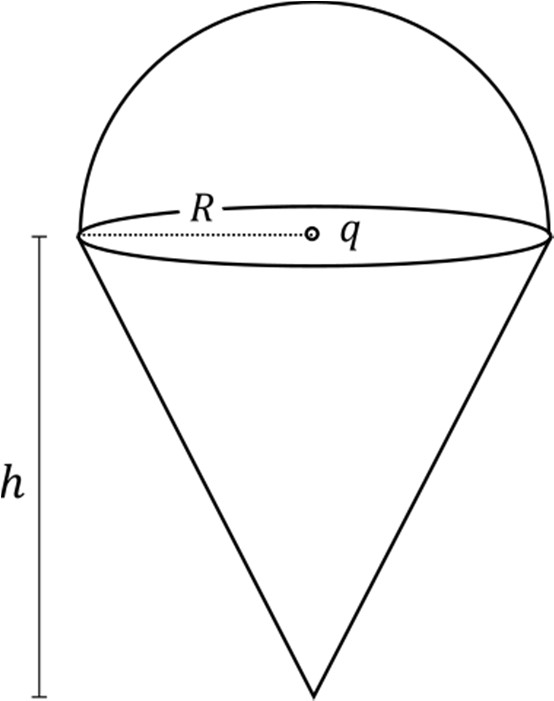
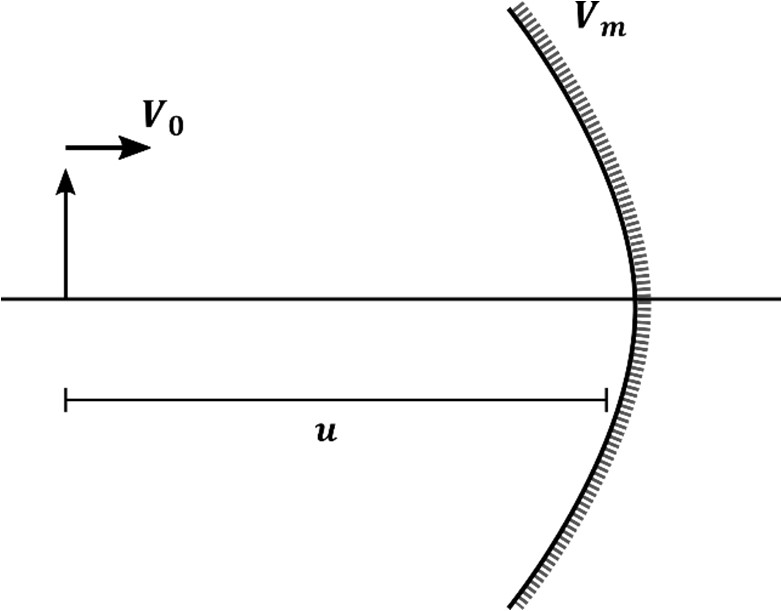
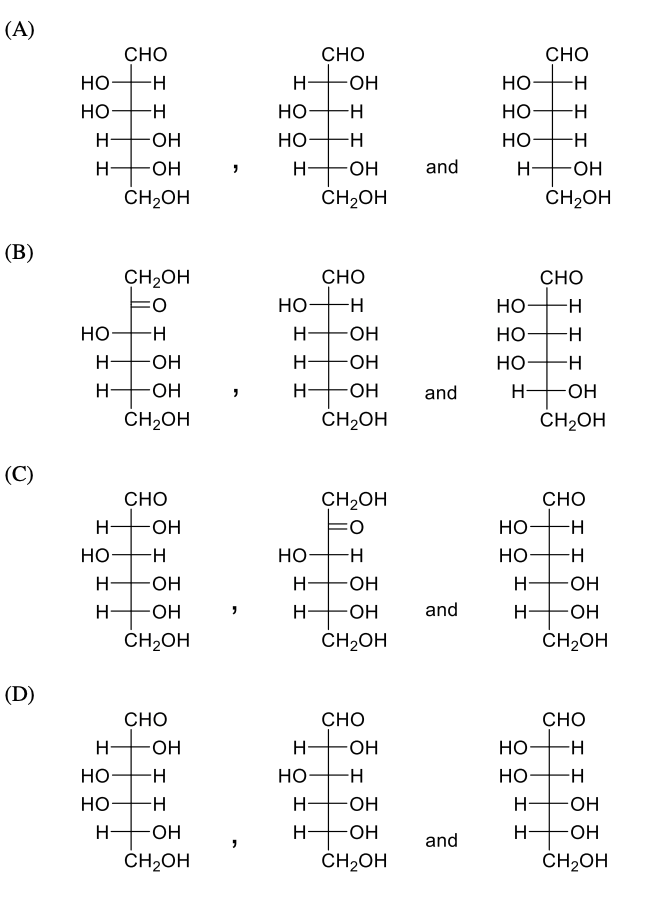
 Which of the following statement(s) is(are) correct?
Which of the following statement(s) is(are) correct?
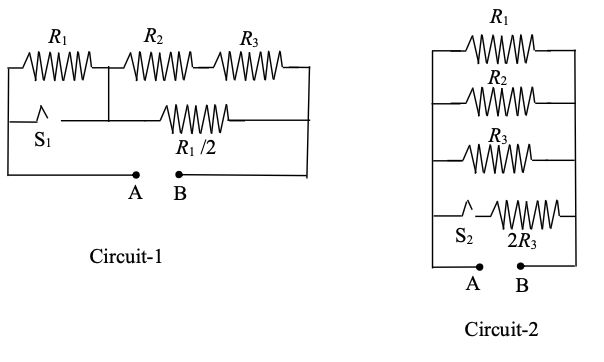 Which of the following statement(s) is(are) correct?
Which of the following statement(s) is(are) correct?
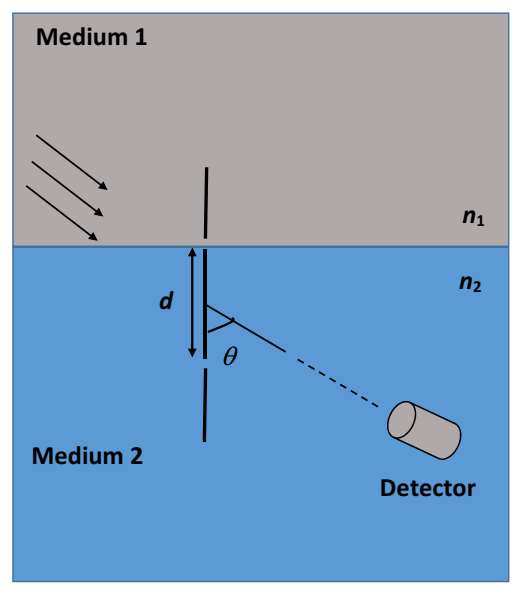 Which of the following statement(s) is(are) correct?
Which of the following statement(s) is(are) correct?
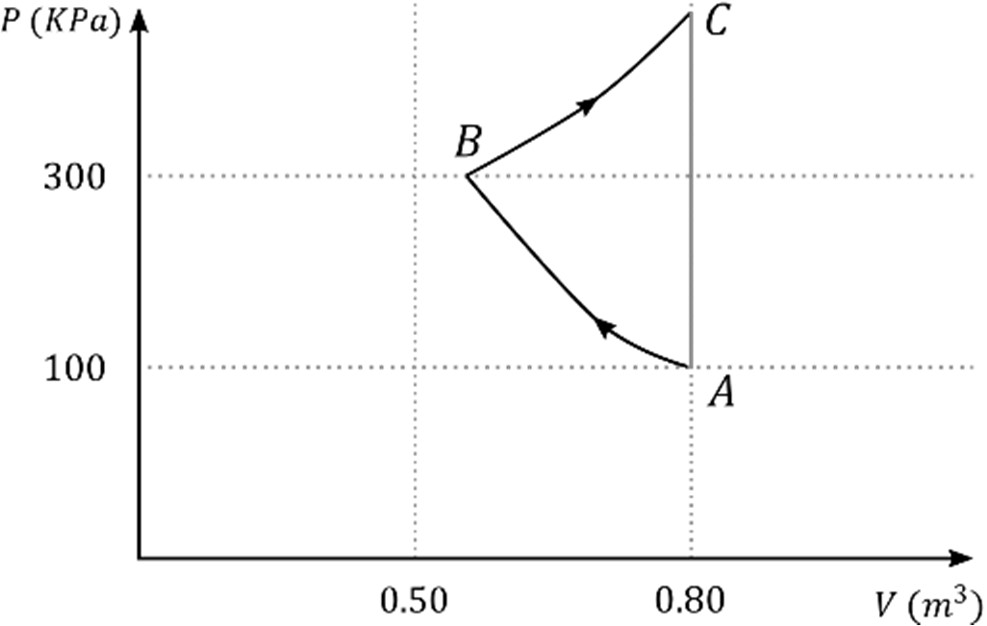 Which of the following statement(s) is(are) correct?
Which of the following statement(s) is(are) correct?